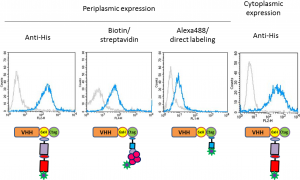
The histidine tag
The DNA sequence specifying a chain of six to nine histidine residues is frequently used in vectors for the production of recombinant proteins. The result is the expression of a recombinant protein with a C-terminal 6xHis-Myc-tagged Recombinant or poly-His tag fused to its N- or C-terminus. Expressed His-tagged proteins can be easily purified and detected because the chain of histidine residues binds to various types of immobilized metal ions, including nickel, cobalt, and copper, under specific buffer conditions.
In addition, anti-His tag antibodies are commercially available for use in assay methods involving His-tagged proteins. In either case, the tag provides a means to specifically purify or detect the recombinant protein without a protein-specific antibody or probe.
Product name: Recombinant human c-Myc protein (tagged)
Purity: > 85% SDS-PAG.
Expression system: Escherichia coli
Accession: P01106
Protein length: full length protein
Animal-free: No
Nature: recombinant
Species: Human
Predicted Molecular Weight: 49kDa
Amino acids: 1 to 439
Labels: Your N-Terminus Tag
Additional sequence information
His-tag N-terminal and Myc-tag C-terminal.
Immobilized Metal Affinity Chromatography (IMAC)
Supports, such as agarose beads or magnetic particles, can be derivatized with chelating groups to immobilize desired metal ions, which then function as ligands for binding and purification of biomolecules of interest. This basis for affinity purification is known as immobilized metal affinity chromatography (IMAC). IMAC is a widely used method to rapidly purify polyhistidine affinity-tagged proteins, resulting in 100-fold enrichments in a single purification step.
The most commonly used chelators as ligands for IMAC are nitrilotriacetic acid (NTA) and iminodiacetic acid (IDA). Once the IDA-agarose or NTA-agarose resin is prepared, it can be “loaded” with the desired divalent metal (eg, Ni, Co, Cu, and Fe). Using nickel as an example metal, the resulting affinity support is often referred to as a Ni-chelate, Ni-IDA, or Ni-NTA resin. The particular chemistry of the metal and the chelation of support determine its binding properties and its suitability for specific IMAC applications.
Affinity purification of His-tagged fusion proteins is the most common application for metal chelate supports in protein biology research. Nickel or cobalt metals immobilized by NTA chelation chemistry are the systems of choice for this application (see next section). In addition, the different varieties of agarose resin provide ideal supports for the purification of His-tagged proteins on a very small scale (96-well filter plates) or on a large scale (array of chromatography cartridges in an FPLC system).
When packed into suitable columns or cartridges, resins such as Ni-NTA Superflow Agarose allow purification of 1 to 80 milligrams of His-tagged protein per millilitre of agarose beads. Compared to cobalt and other ligands used for IMAC, nickel provides a higher capacity for the purification of His-tagged proteins. Thermo Fisher Scientific offers HisPur Ni-NTA Superflow Agarose that exhibits high dynamic binding capacity at a variety of flow rates, making it an excellent choice for large-scale purifications.
Poly-His tags bind best to IMAC resins under near-neutral buffer conditions (physiological pH and ionic strength). A typical binding/wash buffer consists of Tris buffer saline (TBS) pH 7.2, containing 10-25 mM imidazole. The low concentration of imidazole helps prevent nonspecific binding of endogenous proteins that have histidine groups. (In fact, antibodies have histidine-rich groups and can be purified using a variation of IMAC chemistry.)
High salt concentrations and certain denaturants (eg, chaotropic such as 8M urea) are compatible, making sample purification possible in various starting buffers. For this reason, the His tag is best used for the design and expression of recombinant proteins that may need to be purified in denatured form from inclusion bodies. (Compare this to the GST tag, which is an enzyme that must remain functional to allow purification.) It is important to note that EDTA and reducing agents such as DTT and TCEP can negatively affect the performance of regular Ni-IMAC supports upon removal. of the metal.
But specially designed Ni-IMAC chemistry is available that can tolerate the presence of reducing and chelating agents such as EDTA at higher concentrations without loss of performance. EDTA compatible Ni-IMAC chemistry is available in the magnetic bead (Cat. No. A50588) and resin (Cat. No. A50585) formats. They are specifically suitable for purifying expressed His-tagged proteins that are secreted into cell culture media, or for purifying intracellular His-tagged proteins that require the presence of EDTA to maintain stability and function.
Elution and recovery of captured His-tagged protein from an IMAC column are achieved by using a high concentration of imidazole (at least 200 mM), low pH (eg, 0.1 M glycine-HCl, pH 2.5) or an excess of strong chelating agents (eg EDTA). Imidazole is the most common eluting agent. Note that immunoglobulins are known to have multiple histidines in their Fc region and can bind to IMAC supports. High background and false positives can occur if binding conditions are not stringent enough (ie, with imidazole) and immunoglobulins are abundant relative to the His-tagged proteins of interest. Albumins, such as bovine serum albumin (BSA), also have various histidines and can bind to IMAC supports in the absence of His-tagged proteins in the sample or imidazole in the binding/wash buffer.