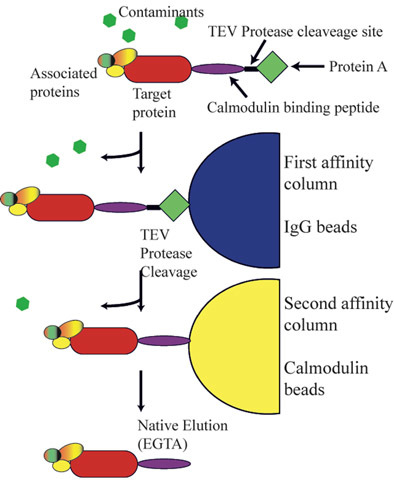
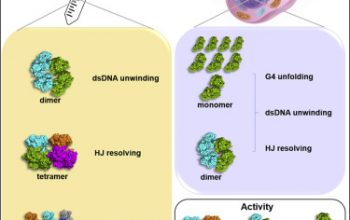
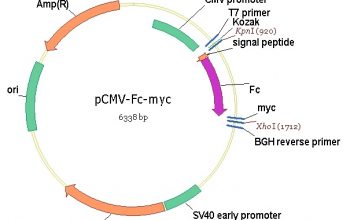
Abstract
Two isoforms of band 3 anion exchanger are expressed in mammalian cells, a residue 911 protein (B3) in red blood cells and a truncated protein (KB3) in the alpha intercalated cells of the kidney. Mutants of both isoforms are known to be associated with human disease, and the mechanism of pathogenesis in several cases has been suggested to be a misselection of mutated proteins but has been difficult to prove. The present study demonstrates the feasibility of using confocal microscopy to investigate the selection of homozygous and heterozygous B3 and KB3 mutants.
K562 erythroleukemia cells offer several advantages for stable expression of B3 but have not previously been used for visualization. A wide range of cell attachment factors, growth conditions, fixation reagents, and primary antibodies was investigated to enable imaging of B3 and endogenous GPA by immunofluorescence confocal microscopy in stable K562/B3 clones. B3 co-localized with GPA on the cell surface and also in an intracellular compartment. Functional cell surface expression of KB3 was also obtained in stable clones of K562.
Importantly, both B3 and KB3 could be expressed as stable green fluorescent protein (GFP) tagged fusion proteins in K562 cells, and N-terminal GFP tagging was shown not to affect B3 targeting or chloride transport properties. or KB3. The use of N-terminal GFP-tagged Recombinant, as well as the double labelling methods developed for immunostaining, will be of great value to investigate the interactions of band 3 with itself and with other proteins during its trafficking in erythroid and kidney cells. This will help elucidate how band 3 mutations can cause human diseases such as hereditary spherocytosis and distal renal tubular acidosis.
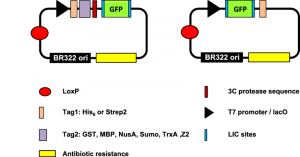
Methods
- Chemicals and materials
Plasmid pEGFP-C3 and pEGFP-N3 DNA were purchased from Clontech Laboratories, Inc. (Pulo Alto, CA, USA). Engine 500 transfection kit and restriction enzymes KphI and HindIII were from Fermentas (Burlington, ON, Canada). Taq DNA polymerase and nucleotide mix were from Invitrogen Canada (Burlington, ON). Plasmid DNA purification kits were from Qiagen. Anti-CD36 antibody was from Immunotech, Beckman Coulter (USA). Stable CHO cell lines and transfected CHO-CD36 cells were grown and maintained.
- Construction of pEGFP-C3-CD36 and pEGFP-N3-CD36
The vectors, pEGFP-C3 and pEGFP-N3, were cut at the cloning sites with restriction enzymes, KpnI and Hind III, and then purified with the Qiagen DNA purification kit. The huCD36 coding sequence was amplified by PCR from pCDNA3-huCD36. For pEGFP-C3-CD36 construction, two primers were used as 5′ ATTAAGCTTATGGGCTGTGACCGGAACTGTG and 5′ ATTGGTACCTTATTTTATTGTTTTCGATCTGCA. Whereas for the construction of pEGFP-N3-CD36, a stop codon at the C-terminus of CD36 was removed using the reverse primer as 5′ ATTGGTACCTTTTATTGTTTTCGATCTGCA.
For both PCR-amplified CD36 products, a Hind III site was created at the N-terminus and a Kpn I site at the C-terminus. Both CD36-PCR products were restriction enzyme cleaved with Hind III and Kpn I and gel purified. Purified CD36-PCR was inserted into GFP-C3 or pEGFP-N3 vectors by normal ligation procedures. Ligated pEGFP-C3-CD36 and pEGFP-N3-CD36 were transformed into Xl-1Blue competent cells and amplified. Recombinant DNAs were sequenced to determine if the CD36 coding sequences were correctly inserted into the vectors.
- Recombinant protein expression and transfection assays
CHO cells were plated on coverslips and plated in 12-well plates in RPM1640 medium overnight to allow cells to grow to approximately 70% confluence at the time of transfection. Recombinant plasmid DNAs, either pEGFP-C3-CD36 or pEGFP-N3-CD36, were largely purified to an OD260/OD280 ratio of at least 1.8 or greater. For transfection, the ExGen 500 in vitro transfection kit (Invitrogen) was used according to the manufacturer’s instructions.
Briefly, 4 µg of pEGFP-C3-Cd36 or pEGFP-N3-CD36 DNA was diluted in 50 µl of 150 mM NaCl mixed with 50 µl of diluted Exgen 500 solution (1:6 with 150 mM NaCl) and incubated for 10 min, and then the mixtures were added to each well with 0.9 ml of fresh RPM1640 medium. Transfected cells were cultured for 48 hours. Expression of the transfected fusion gene was assayed by flow cytometry or confocal microscopy.
- Dil-oxLDL binding assays
After transfection for 48 hours, cells were analyzed for oxLDL binding. For uptake assays, 1 ml of fresh RPM1640 medium was replaced in each well and a designated amount of Dil-oxLDL was added directly to each well and cultured at 37°C for 2 to 4 hours. Cells were then washed twice with PBS and fixed with 4% formaldehyde in PBS for 15 min. After washing several times with PBS, the cells were mounted on slides.
For the surface binding assay, cells after 48 h post-transfection were fixed with 4% formaldehyde for 20 min. After washing three times with PBS, the cells were incubated with Dil-oxLDL at 10 µg/ml in PBS buffer at room temperature for 4 hours. After washing three times, the amount of bound dil-oxLDL was assayed by flow cytometry or confocal microscopy.
- Immunofluorescent staining of cells transfected with anti-CD36 antibody
To test whether recombinant CD36 could be expressed normally, after transfection with pEGFP-C3-CD36 or pEGFP-N3-CD36 for 48 hours, cells were immunostained with the anti-CD36 antibody at 1:100 in blocking buffer (BSA al 1% and 0.1%). % Tween-20 in PBS) at room temperature for 4 hours or at 4 °C overnight. Immediately after primary antibody incubation, cells were washed three times and counterstained with TRIC-conjugated anti-mouse IgG secondary antibody at 1:500 for two hours. After washing three times with PBS, the stained cells were analyzed by flow cytometry or confocal microscopy.
- pRBC adhesion assays
Adhesion assays were carried out as described by Crandall et al (1999) [20]. Transfected cells were fixed in 4% formaldehyde in PBS for 15 min and then rinsed three times with BTS. Adherence assays were carried out using a total volume of 300 µl of infected blood. The erythrocytes were kept in suspension by continuous agitation and incubated with the target cells for 90 min. Adhesion was quantified by direct microscopic observation of pEGFP-C3-CD36 or pEGFP-N3-CD36 transfected cells. Cells transfected with GFP alone were used as a control. Three independent experiments were performed and the data points represent the mean values with the standard error of the mean indicated by the mean bars.